electron withdrawing groups|electron withdrawing effect : Pilipinas An electron-withdrawing group (EWG) is a group or atom that has the ability to draw electron density toward itself and away from other adjacent atoms. This electron density transfer is often achieved by resonance or inductive effects. Electron-withdrawing groups have significant impacts on . Tingnan ang higit pa Ang mga panaginip tungkol sa mga Ahas ay nauugnay sa mga hamon ng buhay. Taliwas sa opinyon ng publiko, ang mga panaginip ay hindi palaging nagdadala ng negatibong kahulugan. . 111 numero ng anghel. 222 numero ng anghel. 333 numero ng anghel. 444 numero ng anghel. 555 numero ng anghel. 666 numero ng anghel. 777 .
PH0 · inductive electron withdrawal
PH1 · electron withdrawing inductive effect
PH2 · electron withdrawing groups list
PH3 · electron withdrawing effect
PH4 · electron donating effect
PH5 · electron donating and withdrawing groups
PH6 · electron donating ability
PH7 · diels alder stereochemistry
PH8 · Iba pa
On this page, you will find the best Paladin Decks to play! This page is updated multiple times a week with Top Legend Decks!
electron withdrawing groups*******An electron-withdrawing group (EWG) is a group or atom that has the ability to draw electron density toward itself and away from other adjacent atoms. This electron density transfer is often achieved by resonance or inductive effects. Electron-withdrawing groups have significant impacts on . Tingnan ang higit pa
Effects on Bronsted acidityElectron-withdrawing groups exert an "inductive" or "electron-pulling" effect on covalent bonds. The strength of the electron . Tingnan ang higit paElectron-withdrawing groups are the opposite effect of electron-donating groups (EDGs). Both describe functional groups, however, . Tingnan ang higit paelectron withdrawing groups electron withdrawing effect• Electron-donating group Tingnan ang higit pa The more electron-rich the aromatic ring, the faster the reaction. Groups that can donate electron density to the ring make EAS reactions faster. If a substituent . Electron withdrawing groups have an atom with a slight positive or full positive charge directly attached to a benzene ring. Examples of electron withdrawing .Learn the definition, examples and effects of electron withdrawing groups (EWG) and electron donating groups (EDG) in organic chemistry. Find out how they influence .
In electrophilic aromatic substitution reactions, existing substituent groups on the aromatic ring influence the overall reaction rate or have a directing effect on positional isomer of the products that are formed. An electron donating group (EDG) or electron releasing group (ERG, Z in structural formulas) is an atom or functional group that donates some of its electron density into a conjugated π system viaCarbonyl groups are electron-withdrawing by inductive effects, due to the polarity of the C=O double bond. It is possible to demonstrate in the laboratory that carbocation A below is more stable than carbocation B, .
electron withdrawing groupselectron withdrawing substituents increase the Lewis acidity of acidic sites by making those sites more electron deficient while electron donating substituents tend to decrease Lewis acidity by making sites less .Learn how an electron withdrawing group (EWG) to a benzyl cation destabilises it, and how the position of the EWG affects the stability. Watch a video created by Shahzad Karim and see .
Learn how substituents affect the reactivity of aromatic rings in electrophilic substitutions. This web page is part of a free textbook on organic chemistry, but it has a glitch and .Learn Electron Withdrawing Groups with free step-by-step video explanations and practice problems by experienced tutors.
EWG, electron-withdrawing group. Full size image. Radical philicity and HAT chemistry. Radical philicity can influence the outcome of many types of organic reactions, .Because Lewis acid-base reactions involve electron donation and acceptance at particular sites, substituent groups which alter the electron density at a site through inductively donating or withdrawing electron .electron withdrawing effect In general, Diels-Alder reactions proceed fastest with electron-withdrawing groups on the dienophile (diene lover). Ethylene reacts slowly while propenal, ethyl propenoate, and other molecules .
Effect of an electron withdrawing group in a benzyl cation. In this video, we see that an electron withdrawing group to a benzyl cation destabilises it. However adding it at ortho- and para- destabilises .As described earlier in this section, hydroxyl, alkoxyl, and amino groups have a strong, electron-donating resonance effect that outweighs a weaker electron-withdrawing inductive effect. When phenol is nitrated, for instance, reaction can occur either ortho, meta, or para to the –OH group, giving the carbocation intermediates shown in Figure .

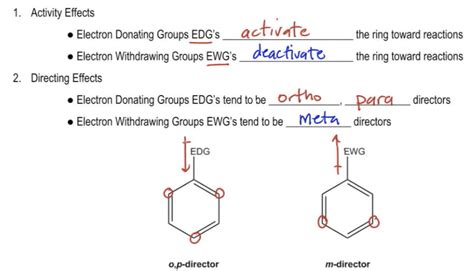
Okay, Whereas many directors electron withdrawing groups, right, they pull electrons out of the ring, so they're gonna tend to add in the meta positions. Okay, I forgot to draw the Dye poll of the electron donating is gonna push electrons into the ring. It directs towards Opie, whereas electron withdrawing groups direct towards the meta positions. In Nucleophilic Aromatic Substitution, an electron-poor aromatic ring is attacked by a nucleophile, resulting in a substitution reaction. The reaction proceeds through a negatively charged (carbanion) intermediate. The reaction is accelerated by the presence of electron-withdrawing groups on the aromatic ring.Here are some general pointers for recognising the substituent effects: The H atom is the standard and is regarded as having no effect. Activating groups increase the rate. Deactivating groups decrease the rate. EDG = electron donating group. EDG can be recognised by lone pairs on the atom adjacent to the π system, eg: -OCH 3.
With our YouTube name generator, all you need to do is enter a few keywords related to what your channel is about, and we'll generate a list of name ideas for you. Generate a Catchy YouTube Name. You want to make a good first impression on YouTube, and one way to do that is to have a catchy name for your channel. A catchy name will help you .You’ll also find midweek Jackpot predictions this weekend (2024), for matches taking place aside from the weekend. Weekend betting is more popular. However, you can still have loads of fun betting on the midweek games. Play Mega Jackpot Today. Now all you need to do is head to your chosen online high stakes bookmaker and choose the Mega .
electron withdrawing groups|electron withdrawing effect